

- Home
- Companies
- isoTracker Solutions Ltd.
- Articles
- ISO 9001: What Is Control of Documented ...
ISO 9001: What Is Control of Documented Information?
Newer versions of this standard have simplified the requirements and group documents and records under one term – documented information.
The ISO 9001 quality standard requires that documented information be maintained, retained, or both.

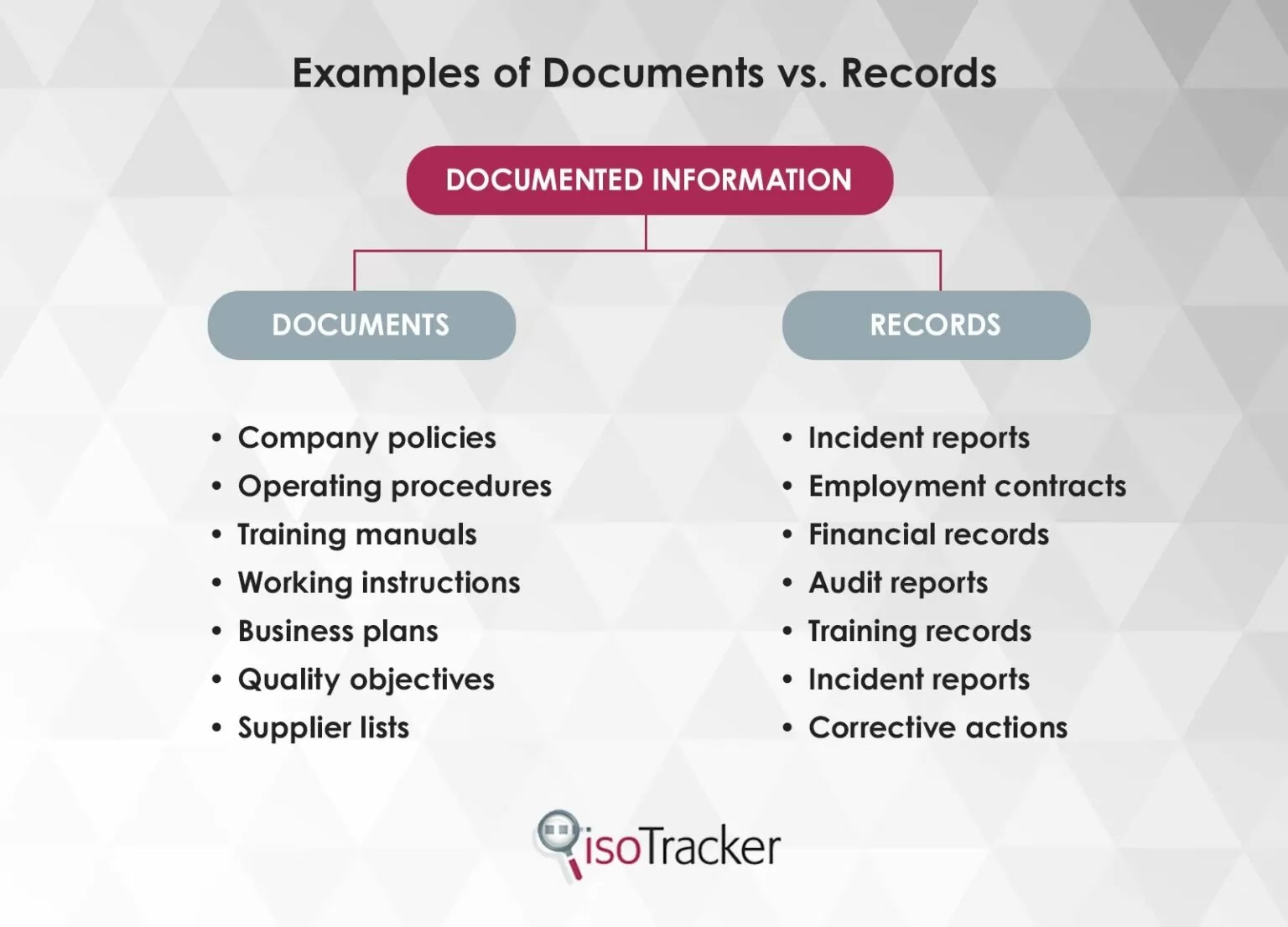
Documented information covers documents and records. In everyday parlance, these two terms are often used interchangeably. Under ISO 9001:2015, however, they have different meanings.
Documents are created to describe what needs to be done. Because this can change, documents are maintained. An example would be a set of procedures.
Records describe what has been done. This cannot change. Accordingly, records are retained. An example might be an audit report.
To give another example, a form is a document. It can be changed and updated as needed i.e. maintained. The form describes an action to be taken, usually supplying information.
Once the form has been filled out, it becomes a record. This record cannot be changed. Another form might be filled out in future to update the information, but the original form will always be retained.
ISO 9001:2015 sets out fewer document requirements than the previous version. Among these are documents containing a quality policy, quality objectives, and the scope of the quality management system.
Documents needed to support the operation of quality management processes should also be maintained even if not required because they may help staff or auditors understand the required documents.
Documents that must be maintained for ISO 9001 compliance include:
- The scope of your quality management system
- Documented information necessary to support the operation of processes
- Your quality policy
- Your quality objectives
The records that ISO 2001:2015 requires will depend on your type of business. See section 4 (c) of ISO’s Guidance on the requirements for Documented Information for a list of the mandatory records to retain.
Examples of records that may be required include:
- Calibration records for any monitoring and measuring equipment
- Records of training, skills, experience and qualifications
- Product or service requirements review records
- Records of design and development of products or services
- Product and service requirements
- Records about customer property
- Change control records
- Record of conformity of product or service with acceptance criteria
- Record of nonconforming outputs
- Results of the quality objective data that is monitored and measured
- Internal audit reports
- Management review meeting minutes
- Results of corrective actions (CAPA)
Once you know what documented information is required, it’s also important to understand the ISO 9001 requirements for controlling this information.
This is covered by paragraph 7.5 of the standard, which states the following requirements:
- Approve documented information for adequacy prior to issue
- Review, update as necessary, and re-approve documented information
- Ensure that changes and current revision status are identifiable
- Ensure relevant versions are available at points of use
- Ensure documented information remains legible and readily identifiable
- Identify external documents and control their distribution
- Prevent obsolete documents from unintended use
- Apply suitable identification if obsolete documents are retained.
Some documented information can be changed or updated (documents). Other documented information doesn’t change (records).
Documents will need to be reviewed, approved, legible, up-to-date, communicated, and readily available.
Records need to be identifiable, stored, protected, retrievable, and retained, but disposed of suitably once obsolete.
isoTracker’s quality management software includes a Document Management module that helps organizations meet all these requirements.
Our modular software is cloud-based, centralized and secure to ensure the safety of your documented information.
It offers these key features:
- a central online repository for documents
- activity history and automated version control
- automated workflow management
- secure archiving of documents
- advanced document security and access control
- automatic task notifications and records of incomplete tasks.
Our quality management software system is available on a subscription basis so there’s no need to pay for modules you don’t need.
Sign up for a free 60-day trial and get access to our full suite of products. See for yourself how the right quality management software can make control of documented information simpler and more efficient.
