

- Home
- Companies
- Amoy Diagnostics Co., Ltd.
- Products
- AmoyDx - HRD Focus Panel

AmoyDx - HRD Focus Panel
The Homologous Recombination Repair (HRR) pathway plays an important role in the repair of double-strand breaks, which is a major cause of cancer development. It has been demonstrated that loss of function of HRR genes, Homologous Recombination Deficiency (HRD) is the inability to repair double strand breaks and will cause a higher risk of developing cancer[1-5]. Patients with HRD showed higher responses to poly ADP ribose polymerase inhibitors (PARPi) and platinum-containing therapies[6-7]. HRD Score testing and BRCA1/2 mutations testing have been approved for patient selection for PARPi therapy.
The AmoyDx® HRD Focus Panel (Reversible Terminator Sequencing) is a next generation sequencing-based in vitro diagnostic test that determines a patient`s HRD status by detecting SNVs and InDels in whole coding regions and intron/exon boundaries of the BRCA1 and BRCA2 genes, and determining a genomic scar score (GSS) using DNA isolated from neutral formalin-fixed paraffin-embedded (FFPE) tissue samples.
The kit is intended to be used by trained professionals in a laboratory environment.
The test kit is based on the Halo-shape ANnealing and Defer-Ligation Enrichment (HANDLE) system technology which is an improved Molecular Inversion Probe (MIP) technology to capture the target gene region (Figure 1). The unique molecular identifier (UID) is introduced to both ends of each DNA fragment to trace back to original template for error correction. The library construction time of HANDLE system is 5 hours with 1 hour hands-on time. After quality control (QC), the prepared DNA libraries are sequenced on Illumina sequencing platforms to detect the BRCA1/2 gene mutations and HRD status.
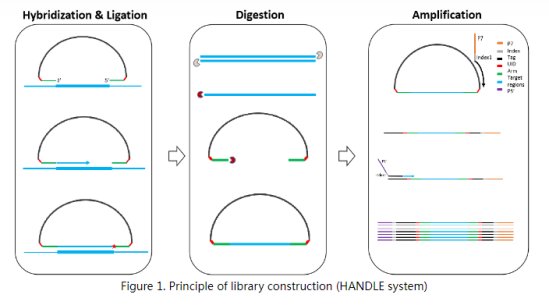
The probe contains an extension arm and a ligation arm which are complementary to the target gene region. First, the extension arm and ligation arm are anchored to the target gene region, and the DNA is extended from the extension arm to the ligation arm using DNA polymerase. Next, the nicks are connected with the ligase to generate the circular products. The remaining linear probes, single-strand and double-strand nucleic acid are digested with the exonuclease. Finally, the universal PCR amplification is performed to enrich the target libraries.
- Accurate evaluation before PARPi therapy- Detect SNV/InDel (BRCA1/2) and Genomic scar score (GSS)
- Automatic and safe data analysis- Onsite server solution for data security
- HANDLE technology (One-Tube workflow)- 5 hours TAT for library preparation with 1 hour hands-on time only
- Limit of Detection (LoD)- SNV/InDel (5%) , 30% tumor content
- Local end-to-end solution- Quick and easy to use in local lab
