

- Home
- Companies
- MicroBioTests Inc.
- Products
- Microbiotests - Model Ostracodtoxkit F ...
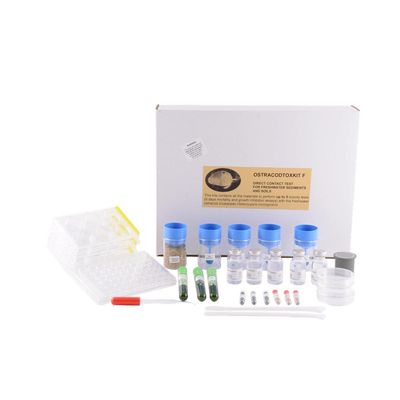
Microbiotests - Model Ostracodtoxkit F -Chronic Direct Contact Sediment Toxicity Test Kit
The chronic direct contact sediment toxicity test is applicable for toxicity screening of contaminated river sediments, soils and solid wastes. Ostracodtoxkit F contains all materials to perform three to five 6-day mortality and growth inhibition tests with the benthic freshwater ostracod Heterocypris incongruens. This sediment-contact bioassay is cost-effective, culture-independent and highly standardised, conform to ISO Standard 14371.
The Heterocypris incongruens toxicity test is a 6-day chronic bioassay based on mortality or growth inhibition of the test organisms, resulting from the direct contact with sediment.
- Tubes with cysts (dormant eggs), which you can hatch on demand within 48h to supply the live biota for the direct contact sediment toxicity test.
- Concentrated hatching and toxicant dilution medium, hatching and test containers, reference sediment, glass micropipettes for the transfer of the organisms, algal food, micrometer slips, wide-mouth pipettes and a microsieve.
- Short Bench Protocol and standard operating procedure brochure with easy-to-follow instructions and detailed illustrations.
- Data scoring sheets and a Quality Control specification sheet with batch number of the cysts, the media, the food and the reference sediment.
All test materials and equipment included in the Ostracodtoxkit F are available separately.
Reproducibility
- We produce high-quality cysts in strictly controlled conditions. This way, we preclude variability associated with recruitment/maintenance of live stocks in conventional bioassays.
- The fixed amount of standard food (live algae) in the test cups precludes variability of results due to food shortage.
- A Quality Control test with a reference sediment is described in detail, so you can easily check accuracy and reproducibility.
Cost-effectiveness
- You can hatch the cysts on demand, which eliminates the need and the costs of continuous culturing and maintenance of your test organisms.
- The direct contact sediment toxicity test requires minimal space and equipment:
- small centrifuge
- dissection microscope
- incubator with lateral lights
- conventional laboratory glassware
- If you store the cysts properly, you can prolong their shelf life for several months and reduce test scheduling constraints.
- You can easily obtain live food (algae) for the test organisms from algal beads included in the kit.
User-friendliness
- Simple handlings and scorings.
- Total performance time of the sediment toxicity bioassay is approximately 2 hours.
- We can provide you with a computer program for easy Toxkit data treatment.
Sensitivity
- The sensitivity of the Ostracodtoxkit F is comparable to the sensitivity of other direct contact sediment toxicity tests with e.g. the amphipod Hyalella azteca and the midge larvae Chironomus riparius.
Validation
- Ostracodtoxkit F is used in many environmental laboratories and research institutes worldwide and is validated by an extensive international interlaboratory comparison.
- You can find a substantial number of publications, posters, reports and reviews for various uses.
- Our cyst-based freshwater sediment toxicity test complies with the protocols of ISO Standard 14371 for regulatory testing.
