

- Home
- Companies
- MicroBioTests Inc.
- Products
- Microbiotests - Model Rapidtoxkit F ...
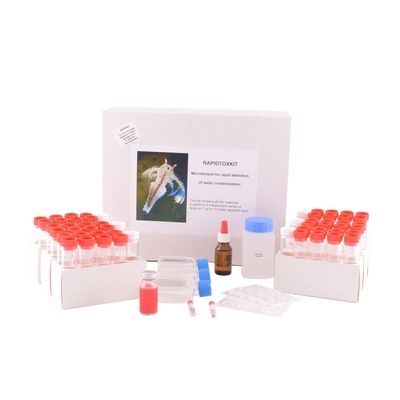
Microbiotests - Model Rapidtoxkit F Thamno -Cyst-Based Thamnocephalus Platyurus Toxicity Test
The cyst-based Thamnocephalus platyurus toxicity test is intended for rapid toxicity screening of chemicals, effluents, surface and groundwaters, wastewaters. In Rapidtoxkit F Thamno, you find all necessary materials to perform 3 independent series of 1h sub-lethal effect ingestion tests with the freshwater crustacean Thamnocephalus platyurus on seven up to fifteen water samples. This cost-effective, culture independent, standardised bioassay conforms to ISO Standard 14380. (Annex A)
Test Criterion
The freshwater Thamnocephalus platyurus rapid toxicity test is a 1h bioassay based on the determination of the uptake of coloured microspheres by the test organisms in a non-toxic medium as compared to the uptake (or absence of uptake) in the test solution under analysis.
- Tubes with cysts (dormant eggs), which you can hatch on demand within 30 – 35h to supply the live biota for the rapid Thamnocephalus platyurus toxicity test.
- Hatching and control water medium, coloured microspheres,hatching and test containers, fixative and observation plates.
- Short Bench Protocol and standard operating procedure brochure with easy-to-follow instructions and detailed illustrations.
- Data scoring sheets and a Quality Control specification sheet with batch number of the cysts and the media.
All test materials and equipment included in the Rapidtoxkit F Thamno are available separately.
Reproducibility
We produce high-quality cysts in strictly controlled conditions. This way, we preclude variability associated with recruitment/maintenance of live stocks in conventional bioassays.
A Quality Control test with a reference chemical can performed, so you can easily check accuracy and reproducibility.
Cost-effectiveness
- You can hatch the cysts on demand, which eliminates the need and the costs of continuous culturing and maintenance of your test organisms.
- The rapid Thamnocephalus platyurus toxicity test requires minimal space and equipment:
- dissection microscope
- incubator with lateral lights
- conventional laboratory glassware
- If you store the cysts properly, you can prolong their shelf life for several months and reduce test scheduling constraints.
User-friendliness
- Simple handlings and scorings.
- Total performance time of the rapid Thamnocephalus toxicity assay is approximately less than 2 hours.
- We can provide you with a computer program for easy Toxkit data treatment.
Sensitivity
- The 1h Thamnocephalus platyurus toxicity test is a very sensitive sub-lethal effect assay for chemical compounds and biotoxins.
- Comparative studies on chemicals and cyanotoxins on the sensitivity of the sublethal effect criterion after 1h exposure versus mortality criterion after 24h exposure revealed a positive correlation between 1h EC50 and 24h LC50.
Validation
- Rapidtoxkit F Thamno has been positive evaluated by US EPA in the framework of the Environmental Technology Verification Program (ETV).
- You can find a substantial number of publications, posters, reports and reviews for various uses.
- Our small-scale freshwater rapid Thamnocephalus platyurus toxicity test strictly adheres to the protocol of ISO Standard 14380.
It allows you to perform 3 independent series of 1h ingestion tests with the freshwater crustacean Thamnocephalus platyurus on 7 up to 15 water samples.
