- Home
- Companies
- Thermo Fisher Scientific
- Products
- Orbitrap Exploris - Model MX - Liquid ...

Orbitrap Exploris - Model MX -Liquid Chromatography Mass Spectrometry (LC-MS)
- Sequence confirmation
- Peptide monitoring
- Intact protein analysis (from small to large molecules, reduced mAbs, and intact mAbs under native and denaturing conditions)
- Glycan profiling
- Oligonucleotide and oligonucleotide impurity mass confirmation
Simplify tasks and reduce errors for more ‘right first time’ analyses with Chromeleon CDS, with built in methods, control, data processing, and management. The suite of smart tools makes your work faster and easier, while ensuring reproducible, high-quality mass monitoring.
High uptime boosts productivity. Rapid “set and forget” calibration procedures provide consistent mass stability for at least four weeks at prescribed conditions. Instrument status monitoring and optimized pressure control alert users when maintenance is needed, avoiding unnecessary downtime and repeat analyses.
Designed for data integrity, data security, and compliance, fully scalable Chromeleon CDS provides comprehensive technical controls to give you a competitive edge in meeting global regulatory requirements.
Robust and reliable performance from system to system and site to site ensure confidence in results used to make critical decisions. Industry-proven Orbitrap technology, now purposely designed for mass monitoring in future-proof lab operations.
Easy transfer of sophisticated Multi-Attribute Method (MAM) assays from development to manufacturing bridges the gap between development and QC.
The dedicated, global Thermo Scientific MAM team maximizes your productivity and confidence with installation, training, and fast service and support. Standardized system performance evaluation test (SET) and installation qualification/operational qualification (IQ/OA) thoroughly assess system performance with comprehensive installation and post-maintenance acceptance criteria.
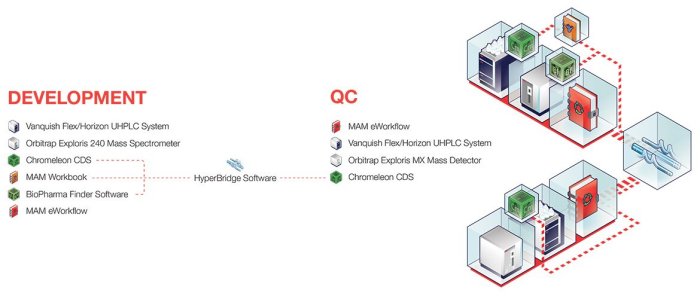
Benefit from the end-to-end Thermo Scientific MAM 2.0 workflow approach to comprehensive characterization and monitoring of biotherapeutics from development to commercialization. Orbitrap Exploris 240 mass spectrometer methods, established during development, seamlessly transfer to the Orbitrap Exploris MX detector in manufacturing or by contract partners. In addition to full integration into the high-resolution MAM 2.0 workflow, the usability and low cost of the Orbitrap Exploris MX detector make it the perfect fit for high-throughput QC and contract labs.
Users can identify and evaluate relevant product quality attributes (PQAs) via high-throughput analytics provided by Thermo Scientific BioPharma Finder software. Connected labs can access data from development through QC using MAM 2.0 powered by Thermo Scientific HyperBridge software. And, PQAs can be monitored throughout the entire drug development process using the MAM target peptide workbook built from the list of identified peptides upon incorporation into a Chromeleon processing method. The processing method along with the instrument method, injection sequence, view setting and report template can be stored into a MAM eWorkflow for easy and direct transfer to QC via Chromeleon CDS Enterprise software.
- Fast decision making based on high-confidence PQAs obtained using industry-leading HRAM Orbitrap technology
- Seamless knowledge and method transfer across instruments, departments, and sites using compliance-ready, Chromeleon CDS enterprise software, and HyperBridge server or cloud