

- Home
- Companies
- Ruiyuan Group Limited
- Products
- Ruiyuan - Palladium (II) Chloride ...

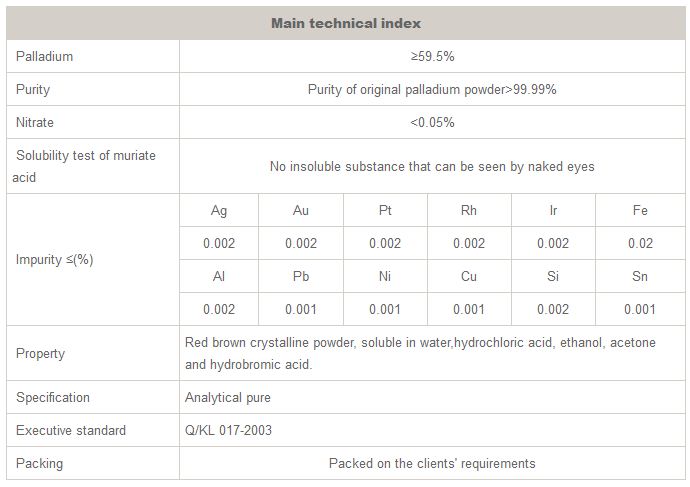
Ruiyuan - Palladium (II) Chloride (PdCl2)
Cas No.: 7647-10-1, Molecular formula: PdCl2, Molecular weight: 177.31, Synonyms: Dichloropalladium, Palladium chloride, Palladous chloride, Palladium dichloride, Palladium(II) chloride, Pd(II) chloride, Enplate activator 440, Palladium(2+) chloride, Palladium chloride (PdCl2), Palladium (II) chloride, PALLADIUM CHLORIDE (2+), CCRIS 6263, WLN: PD G2, HSDB 4362, 14814_RIEDEL, 205885_ALDRICH, 283606_ALDRICH, 323373_ALDRICH, 520659_ALDRICH, NCI-C60184,EINECS 231-596-2,HSDB 4362,NSC 146183,Palladium chloride(2+)
Two forms of PdCl2 are known. In both forms, the palladium centres adopt the square-planar coordination geometry that is characteristic of Pd(II). Furthermore, in both forms, the Pd(II) centres are linked by μ2-chloride bridges. The α-form of PdCl2 is a polymer, consisting of "infinite" slabs or chains. The β-form of PdCl2 is molecular, consisting of an octahedral cluster of six Pd atoms. Each of the twelve edges of this octahedron is spanned by Cl-. PtCl2 adopts similar structures, whereas NiCl2 adopts the CdCl2 motif, featuring hexacoordinated Ni(II).[1]
Evolution of β-PdCl2 structure: Start with cubic lattice, remove corner and centered lattice points, inscribe octahedron (red lines), label corners as X (twelve Cl- centers) and face-centered atoms as M (six Pd(II) centers).
Palladium(II) chloride is a common starting point in the synthesis of other palladium compounds. It is not particularly soluble in water or non-coordinating solvents, so the first step in its utilization is often the preparation labile but soluble Lewis base adducts, such as those derived from acetonitrile or benzonitrile.[2]
The acetonitrile complex is prepared by treating PdCl2 in refluxing acetonitrile:
PdCl2 + 2 MeCN → PdCl2(MeCN)2
Although occasionally recommended, inert-gas techniques are not necessary if the complex is to be used in situ.
Even when dry, palladium(II) chloride is able to rapidly stain stainless steel. Thus, palladium(II) chloride solutions are sometimes used to test for the corrosion-resistance of stainless steel.[citation needed] Palladium(II) chloride is sometimes used in carbon monoxide detectors.
The related nickel and platinum compounds are known to be irritants of the skin and the respiratory system and, in some cases, carcinogenic, and its is generally accepted as prudent to assume that palladium compounds share these risks.
