Syphilis rates are increasing around the globe and symptomatic or visual lesion diagnostic approaches may be missing some cases presenting as an atypical lesion. Molecular diagnostics are recommended for management of genital lesions to improve diagnostic accuracy and subsequent patient management.
- Ulcerations or lesions in the ano-genital and oral regions can be caused by a variety of bacterial and viral infectious agents.
- Syphilis lesions can present atypically, be painful, and appear indistinguishable from herpes.
- Up to 3% of genital lesions may be atypical zoster (VZV) presentations.
Streamline your workflow and increase productivity for rapid, routine diagnostics. Full automation options include sample and qPCR set-up, through to validated software solutions for automated result calling and simple data processing.
- Detect all four common lesion-causing targets or choose to detect herpes and varicella only.
- Validated on genital and non-genital samples
SpeeDx analysis software is included in contract pricing, is license free, and installed on a high security and GDPR compliant platform. Designed to support the IVD laboratory environment, features include:
- audit trails
- user traceability
- LIS connectivity
- QA and batch management
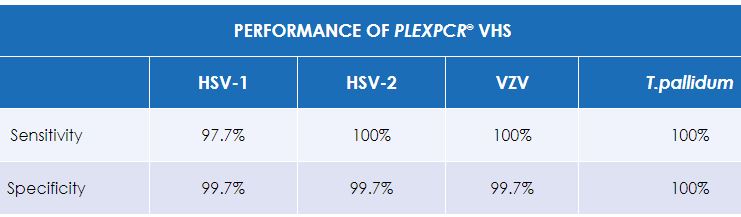
The PlexPCR® VHS kit is a qualitative real-time PCR (qPCR) assay for the detection of Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2), Varicella zoster virus (VZV) and Treponema pallidum.
Single well: Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2), Varicella zoster virus (VZV), Treponema pallidum (Syphilis) & Internal Control
Sample types- Swabs: genital, non-genital, anal/rectal and oral
- Applied Biosystems® 7500 Fast (7500 Fast)
- Roche LightCycler® 480 Instrument II (LC480 II)
- Bio-Rad CFX96™ IVD (CFX96 IVD)
- Bio-Rad CFX96™ CFX96 Touch™ (CFX96 Touch)
Products are shipped on dry ice or ice gel packs.
Storage & stabilityExpiry dates are stated on the labels. It is recommended that freeze/thaw cycles be limited to less than 15. Store protected from light at – 20°C.
Intended useFor in vitro diagnostic use. Not for sale in the USA.
Regulatory statusCE-IVD, TGA cleared