

CellFE - Scalable, High Throughput Microfluidic Technology
CellFE has developed a scalable, high throughput microfluidic technology for the efficient delivery of gene-editing molecules into cells. CellFE’s technology uses rapid cell compressions to induce a unique behavior causing cells to exchange fluid with the environment and uptake large molecules. The technology has demonstrated the delivery of mRNA, CRISPR Cas9 protein, and plasmid DNA without a negative impact on proliferation, immunophenotype, and exhaustion of the transfected cells.
CellFE’s device induces rapid compressions on cells, causing temporal loss of intracellular fluid and cell volume decrease. Following the compression, cells recover volume and actively uptake fluid and materials from their surroundings. This convective transport mechanism is extremely effective in carrying target material into the cell ‘s interior, with a low impact on viability. This process occurs in milliseconds as opposed to seconds for other technologies and does not require an incubation period.
- Unlimited in cargo type including large sizes (>10kB)
- Maintains viability (%90)
- Highly efficient (e.g. >80 mRNA)
- Scalable from R&D into clinic
- Effective for variety of cells (including adherent)
A world where gene therapies are the standard - and not the exception - in healthcare.
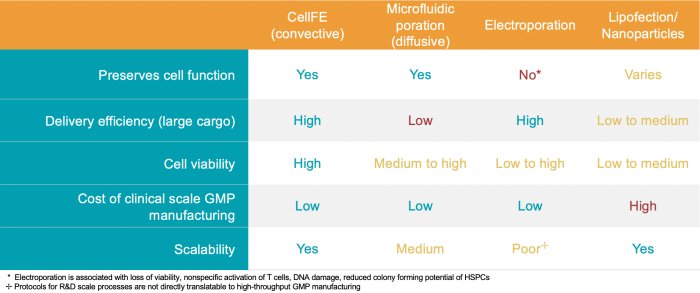
Greater yield of functional, healthy cells
CellFE’s technology enables the cGMP manufacturing of cell therapies in a fundamentally different way to substantially increase product yield and quality. Manipulation of cells in our device results in no observable stress on sensitive cell types, improving quality and reducing regulatory risk. In contrast, electroporation often suffers from loss of cell function which may limit the efficacy of therapies.
Simple scaleup accelerates path to clinic
Unique to microfluidics, all cells are treated uniformly, unlike the uneven permeabilization common with electroporation. An innovative multiplexed chip design makes it possible to transition from an R&D, multi-well plate format to clinical scale. A simple change of the chip is required without any process parameter changes. Unlike traditional methods, product quality does not change with scale.
Simple scaleup accelerates path to clinic
Unique to microfluidics, all cells are treated uniformly, unlike the uneven permeabilization common with electroporation. An innovative multiplexed chip design makes it possible to transition from an R&D, multi-well plate format to clinical scale. A simple change of the chip is required without any process parameter changes. Unlike traditional methods, product quality does not change with scale.
