VZV and HSV have similar clinical presentations but require different patient management. Guidelines around the world recommend molecular testing to improve accuracy of ano-genital ulcers and ensure appropriate patient management.
- Assist patient management with definitive diagnostic tool
- HSV-1 is increasingly prevalent in genital infections
Streamline your Herpes testing workflow with a simple, high-performance molecular testing solution.
- Single-well multiplex test
- Validated on range of specimen types
- No auxiliary software required
Simplify your herpes testing workflow.
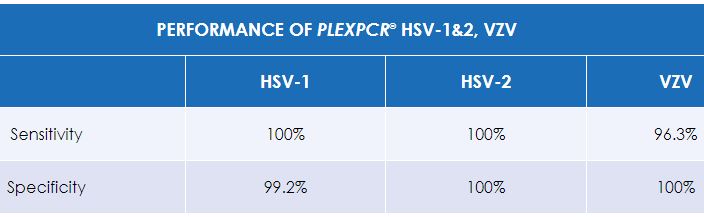
The PlexPCR® HSV-1&2, VZV kit simultaneously detects and differentiates Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2) and Varicella zoster virus (VZV) nucleic acids isolated and purified from cutaneous or mucocutaneous lesion swab specimen.
Single well: Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2), Varicella zoster virus (VZV), Internal Control
Sample types- Swabs: genital, non-genital, anal/rectal and oral
- Roche LightCycler® 480 Instrument II (LC480 II)
Products are shipped on dry ice or ice gel packs.
Storage & stabilityExpiry dates are stated on the labels. Excessive freeze/thawing is not recommended. Store protected from light at – 20°C.
Intended useFor in vitro diagnostic use. Not for sale in the USA.
Regulatory statusCE-IVD, TGA cleared