Qualio
Most popular related searches
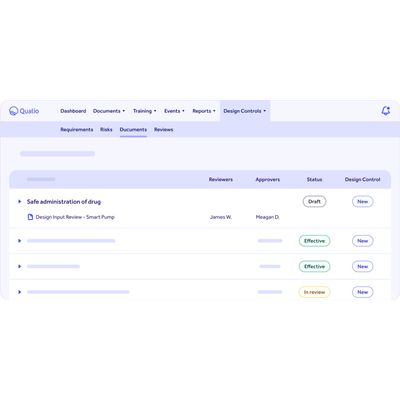
A smarter way to manage design controls
Challenge
- Quality teams work in one system. Engineering teams in another.
- Siloed and disconnected teams and platforms make traceable, compliant product development impossible to manage.
- Work grinds to a halt, quality and development work against each other, and your path to market becomes a complex nightmare.
Solution
- Qualio Design Controls offers a single source of truth for all your product development activities.
- Pull product data into Qualio with integrations. Control inputs, requirements and risks. Bring your quality and engineering teams into sync.
- All underpinned by a complete, compliant document stack to show your auditors.
Unite systems and data to enable effective collaboration and stop delays
- Demolish quality and engineering siloes by connecting systems like Jira, Azure DevOps and TestRail to Qualio
- Build a real-time, always-up-to-date document stack that flows seamlessly from your engineering team to quality
- Centralize all design control data by product, from requirements to inputs, outputs, risks and drag-and-drop attachments
Maintain end-to-end traceability across systems in real time
- Easily pinpoint missing tests, risk mitigations and more for a right-first-time approach
- Drive changes and product updates quickly and easily
- Unlock 100% traceability across design elements while your engineering teams work in the source systems they know and love
Generate release documentation with one click
- Easily produce quality documentation to ensure you’re delivering a safe and effective product, eliminating time-consuming paper-based processes and manual governance
- Be 100% audit-ready at all times with documentation, e-signatures and objective evidence
- Access automated snapshots of product documentation for each stage of your product lifecycle
Purpose-built for your quality and product teams
Quality teams
Quality teams
- For medical device, SaMD and SiMD companies enjoy a single source of truth for design controls and product data.
- Export automated documentation, embed FMEA and ISO 14971 risk management and unlock complete traceability from input to output.
- Enjoy uninterrupted development as the engineering systems they`re used to are linked directly to Qualio.
- Develop and innovate, safe in the knowledge that your work is flowing accurately and in real time straight to the quality department.