

- Home
- Companies
- up2LIMS, by up to data GmbH
- Software
- up2LIMS - Laboratory Information ...
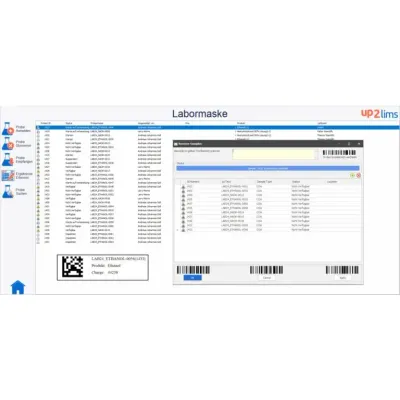
up2LIMS - Laboratory Information Management System (LIMS) Software
up2LIMS is an advanced Laboratory Information Management System (LIMS) designed for mid-sized enterprises, providing a comprehensive suite of functionalities tailored to enhance laboratory operations efficiency. Developed by seasoned laboratory experts with over three decades of experience, it utilizes the proven technology of Thermo Scientific™ SampleManager LIMS™. up2LIMS is capable of supporting flexible order management with customizable order types and integrated barcode functionality ensuring sample traceability. The system supports manual or interface-based data acquisition, integrated document management, and a lab execution system (LES) for structured procedural guidance. Additionally, an integrated statistical quality control module enables accurate process parameter analysis, with features like threshold monitoring and trend analysis. The system also includes an inventory management module for seamless tracking of laboratory substances and reordering triggers. Its electronic lab notebook (ELN) allows for flexible data capture, while seamless integration with laboratory devices and IT systems enhances automation and data management.up2LIMS – One software. All functions. Perfect fit for efficient laboratory work.
Simple and precise to use, scalable to your requirements and successfully implemented in laboratories of all sizes and industries.
- Flexible and individual, even for small and medium-sized laboratories
- Complete process coverage; compliant with ISO 9001:2015 and other legal and regulatory guidelines
- Fast implementation, ready to use
- Fair, transparent pricing
- Personal support from a single source
- Integrated Laboratory Information Management System (LIMS), Electronic Lab Notebook (ELN) and Lab Execution System (LES) included
The individually configurable user interfaces enable fast and intuitive operation. up2LIMS supports the flexible entry of test orders; each order type can be individually configured. After the order has been entered, the test scope can be stored directly. Integrated barcode functions enable transparent sample assignment and complete traceability. Measurement is supported by integrated document management and the Lab Execution System (LES), and work instructions are available for review at the appropriate location. Results can be recorded manually or via direct device interfaces, including functions such as limit value monitoring and change history. An approval process enables multi-stage approvals and an audit level. After approval, certificates of analysis and standard reports can be generated automatically.
As a laboratory manager, up2LIMS offers you customized features for efficient laboratory management. Configurable workflows allow you to maintain a constant overview of the current analysis status and your processes. Individual workflows are optimally mapped to ensure maximum transparency, compliance, and data integrity . The SQC (Statistical Quality Control) module enables the precise calculation and graphical display of process and product parameters, including limit value display and trend analysis. Furthermore, laboratory performance indicators such as throughput times and error rates are always available to you, enabling real-time and data-driven evaluation of your laboratory's efficiency and quality.
Document management in up2LIMS enables the versioned and controlled management of all relevant documents such as work instructions, SOPs, and standards . Documents can be referenced at the appropriate location in the LIMS. This allows an SOP to be assigned to an analysis method, a user manual, or an instrument. Established document management functions such as keyword searches, controlled document processing (versioning, review and approval, audit trail), and electronic signatures are already integrated .
up2LIMS enables the precise recording and management of instruments, reagents, standards, and consumables. This gives you a complete overview of the condition and availability of your instruments, as well as the quantity and expiration date of your reagents. Instrument components, such as pH meter electrodes, can be recorded and managed, including the complete maintenance of an instrument logbook. The planning and execution of maintenance and service activities are fully mapped in the LIMS.
up2LIMS features inventory management, allowing all substances and stocks to be transparently mapped from order to consumption logging. When stock levels fall below a defined level, information for reordering can be sent automatically. Hazard warnings according to various standards such as R/S, GHS, and NFP 704 can be stored for substances, displayed, or printed on labels.
The Lab Notebook (ELN), already integrated into up2LIMS, allows you to easily and securely capture unstructured data. This data can be referenced in the LIMS, allowing direct access to devices and samples at any time. Your Lab Notebook can be flexibly structured according to user or project specifications in a wide variety of formats; images can be inserted or molecular structures referenced. Integrated spreadsheet pages enable calculations and the creation of graphics. All captured data is searchable.
An integrated Lab Execution System (LES) in up2LIMS enables precise, step-by-step execution of various methods such as analyses, instrument calibration, or reagent preparation. It guides you safely through each required step , provides the necessary instructions, and documents the recorded parameters. The tablet app gives you maximum freedom of movement in the lab and allows you to document the execution directly at your workstation.
With up2LIMS, your laboratory achieves the maximum level of automation, as seamless integration into your systems is guaranteed. Furthermore, various interfaces to laboratory devices are already included. up2LIMS supports bidirectional interfaces, i.e., the transfer of sample lists or sequences to the device, including direct retransmission of the results. The received data can, of course, also be processed graphically. Furthermore, you are provided with options for communicating with other IT systems. File-based communication, for example, enables time- or event-controlled export and import of data. Furthermore, you are provided with a wide variety of options for communicating with other IT systems. File-based communication, for example, enables time- or event-controlled export and import of data. Direct read or write access to databases, as well as communication via web services, are also possible.
