

MARRS Assurance - Patented Security Software for Achieving Compliance
MARRS Assurance is patented security software for achieving compliance with electronic regulatory standards, such as 21 CFR Part 11, Good Manufacturing Practices (GMP), Good Automated Laboratory Practices (GALP), and more. MARRS Assurance automatically integrates with any instrument and throughout the entire lab, providing the capability for laboratories to personalize the software in order to meet their lab’s dynamic security needs. A critical element of MARRS Assurance is it’s unique ability to extend the compliance to the actual use of the data, providing a truly secure layer of defensibility of quality assurance that ensures that results used in decisions and studies have the utmost supportive quality. Furthermore, MARRS Assurance can seamlessly integrate within the entire laboratory Quality Assurance Program (QAP) and other compliance systems, and is applicable to various analytical industries.
- Compliance to Regulatory Agency and Industry
- Compliance to Standard Operating Procedures
- Compliance to Analytical Method Requirements
- Compliance to Client Requirements
MARRS Assurance Security:
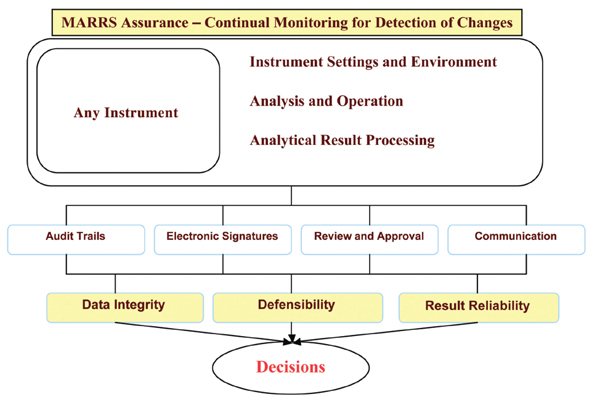
Security and Setup:
MARRS Assurance Security is robust with the capability to self-define and manage Users, User Groups, Privileges, and Access Rights. User Groups can be defined to establish hierarchies of Review and Approval. For example a standard laboratory User Group may consist of Analyst/Technician, Secondary Review, Quality Assurance, and Management/Administrators.
In addition to security, laboratories can personalize the MARRS Assurance Security for:
- Continual Monitoring of instrument files, folders, and tables
- Electronic Signature Types
- Review and Approval
- Types of Review and Approval
- Instant Messaging
- Work Flow/Process Flow definition
Continual Monitoring, Electronic Signatures, Electronic Versioning, and Audit Trails
Continual Monitoring, Electronic Signatures, Electronic Versioning, and Audit Trails Laboratories can continually monitor instrument files, folders, and tables for changes and anomalies to the Instrument Environment, Instrument methods/setup, and analytical data processing. Multiple Audit Trails capture the electronic signatures of User ID, Windows ID, Instrument ID, Review ID, and Approval ID. Electronic Versioning is on-going, providing a roll back feature to review the changes and anomalies. The Electronic Versioning mechanism can also perform an electronic comparison of changes to files or tables in order to pinpoint specific data elements that have changed.
The MARRS Assurance Security provides the following Audit Trails:
- Instrument Environment Audit Trail: monitoring of files, folders, and tables
- Analytical Processing Audit Trail: result changes as data is processed, calculated, reviewed, automated quality assurance, analytical anomalies, data integrity …
- Analytical Review and Approval Audit Trail: Tracks reviews and approvals as the data goes through the laboratory work flow.
Work Flow/Process Flow
The MARRS Assurance Security software allows laboratories to self-define and manage Work Flows/Process Flows for analytical data review and approval steps. For example, a standard laboratory process may contain a series of reviews and actions. Processed analytical data may follow these steps:
- The Analyst/Technician reviewing the analytical data for quality assurance (QA) issues. The Analyst/Technician makes decisions based on the QA and provides analytical notes on decisions, then provides some conclusions.
- The secondary review user picks up the Analyst/Technicians reviewed data and confirms the analytical notes, decisions, and conclusions for approval.
- Specific QA issues and anomalies on the analytical data are sent to the QA Group for review and discussion.
- A completed status is passed on to the Reporting Group
Review Checklists
The MARRS Assurance Security software allows laboratories to self-define and manage Review Checklists to be used in Instrument Maintenance, Instrument Setup, Analytical Review, and Secondary Review. These checklists are then provided as a mechanism to ensure that proper procedures were followed by asking a series of questions of the user until the checklist is complete. The checklist is then electronically stored and provided in the Audit Trails.
Instant Messaging
Each user can setup Instant Messaging profiles to inform them when an action is required, complete, an automated review summary is available, and notification of issues, etc …
These Instant Messages are automatically emailed to the user when a process has occurred that matches the user profile.
Key Benefits:
- Ensures dynamic compliance to electronic regulatory standards for FDA 21 CFR Part 11, CDC, DOE, DOD, EPA, HACCP, ISO, NELAP, USDA, and Nuclear Regulatory Commission in data handling
- Integrates to present laboratory processes with no decrease in production
- Automates and streamlines the quality assurance reviews
- Communicates analytical issues and results throughout the laboratory
- Ensures data quality and integrity
- Total compliance from instrument to client
Add-on Features Available:
- MARRS Assurance Security can be integrated with any other instrument(s)
- Audit Trail integration with other compliance systems
- User Scheduling for analytical studies, instrument maintenance, routine QA checks
- Non-Conformance Reporting and Tracking
- Production Analysis Module
