

Qualio - Risk Management Software
Stress-free audits with risks under control. Proactively identify, mitigate, prevent and resolve risks before they happen.
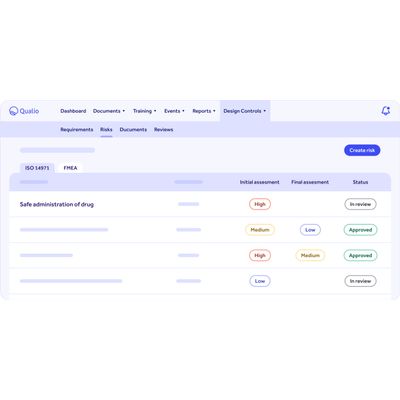
An integrated digital approach to risk
Challenge
Risks lurk in every nook and cranny of your business. Pinpointing and tackling every single one is extremely difficult.
And when you do get a risk management system in place, it doesn`t always integrate with your product design and development processes - leaving crucial risks undetected and unresolved as you get to market.
Solution
Qualio embeds risk management into the heart of your product design and development activities.
Total visibility, bespoke action workflows and automated document stack creation removes the effort and headache of risk management and lets you bring a safe, compliant product to your patients and customers.
- Build a single source of truth for risk identification, monitoring and remediation
- Save time and money by identifying risks early in product development
- Align your quality and product teams around risk and vulnerability activities
- Define your risk criteria then apply them across your whole quality system
- Link risks to your product development design control and documentation processes to squash risks for a right-first-time approach
- Seamlessly access risk analysis and traceability matrices with one click
- Build automatic ISO 14971 and FMEA compliance into your medical device design controls
- Show complete risk control to your auditors
- Export risk records and link risks to CAPA plans and Qualio documents
