

- Home
- Companies
- UL Solutions LLC- WERCS Studio
- Software
- UL-Solutions - Version ComplianceWire - ...
UL-Solutions - Version ComplianceWire -Learning and Qualification Management System
Navigate FDA regulations, automate training and implement risk management to support the development of safer and more effective products.
Medical device and pharmaceutical manufacturers trying to keep up with ever-changing regulations have a tough job. Medical technology advances quickly, and with it the need for compliance systems to support patient safety. To help you and your workforce stay up to date with the latest U.S. Food and Drug Administration (FDA) regulations, Good Clinical Practice (GCP) training, HIPAAA training, ISO 14971 training and more, we created ComplianceWire®.
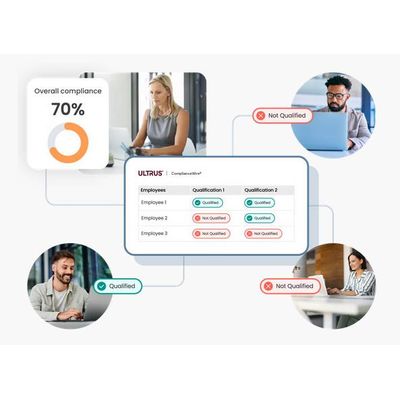
Track and monitor the status of your employees in meeting their required qualifications and training in aggregate, or drill into the areas where improvement possibilities and risks are identified.
Dashboards and reports provide an organization-wide view of compliance and qualification status.
Drill down into each team and qualification in real time to understand exactly what is missing that is preventing compliance.
Schedule reports to team leaders to support the active promotion of training and qualifications.
Easily communicate with and monitor your employees and follow up on post-audit findings (CAPAs) and other regulatory obligations. Upload required training, qualifications and policies to maintain compliance.
Easily create your own courses or edit and re-brand our off-the-shelf library to cater the content to your organization.
Users can easily engage in dynamic eLearning courses to scale up training.
Create different mechanisms for standards to meet compliance, build out team hierarchies and integrate with your wider ecosystem.
ComplianceWire is natively compliant with FDA 21 CFR Part 11 standards and contains many of the requested reports from regulatory agencies right out of the box. Respond to authorities and internal audits confidently.
Learn how UL Solutions verifies every major release of ComplianceWire®, with insights from our Strategic Advisory experts.
Leverage the ComplianceWire team to prepare for and support regulatory inquiries.
ComplianceWire is regularly audited internally and by our customers. On average, our customers audit ComplianceWire over 50 times a year, including paper, remote and onsite audits.
Grow expertise with ComplianceWire’s e-Learning Library for Life Sciences
Get help to remain compliant with the U.S. Food and Drug Administration (FDA) regulation of medical devices and pharmaceuticals. Our course catalog contains more than 400 life sciences e-learning courses, many of which were co-developed with the FDA.
UL Solutions offers over 1,000 standard regulatory and knowledge-focused e-learning courses, including 21 CFR part 820 training, 508 compliance e-learning, CAPA training and more.
UL Solutions expertise combined with FDA-authored and/or reviewed courses, identical to those used by the FDA to train its inspectors and investigators.
Includes stylized topic-specific videos, embedded interactions, and quizzes, all implemented by skilled instructional designers.
Elevate quality from being a manufacturing-only focus to being a central pillar that helps reduce risk across the entire business.
Learn how ComplianceWire can help support qualification and quality across your supply chain.
Support positive patient outcomes and help clinical research by covering qualifications across clinical trials.
Help support employees, suppliers, partners and vendors who are meeting the standards of excellence associated with your brand.
- Quick and easy-to-learn intuitive interface
- Automated population of SDS in Sections 2,3,8,11,12,13,15and16
- Regulatory content from more than 100 global lists
- Standard US and EU/CLP SDS templates
- Multilingual phrase library with more than 21,000 phrases for instant SDS translation into 45+ languages
- Automatic PDF secure document creation with immediate Web availability
- Integration with a database of a quarter million raw materials
- Global GHS classification module and transportation classification module
- Automated European product classification (includes R- and S-phrases and Annex I Data)
- Automated analysis of product components against current regulations Webviewer
- LabelWERCS Designer Module • Print station
- EU, GHS, WHMIS and Transportation Wizards
- Automatic PDF secure document creation with internet and intranet distribution
- Seamless updates
