E-Scan - Model 655 -Offline Micro Leak Test Instrument
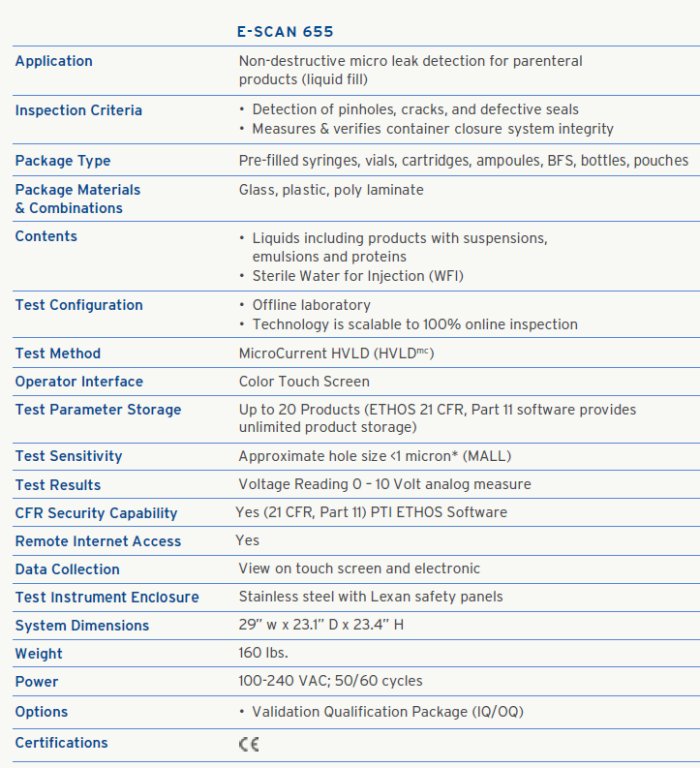
MicroCurrent HVLD technology is the optimal solution for all parenteral and biologic products. The E-Scan 655 is a revolutionary deterministic offline micro leak test instrument that utilizes a new class of HVLD technology to inspect vials, syringes, and other liquid filled parenteral products for container closure integrity.
The E-Scan 655 technology is a MicroCurrent conductivity test method, HVLDmc , that is completely non-destructive to the container and product; exposing the package and product to lower voltage than other conductivity based solutions. The technology uses a non-contact and non-invasive test method that requires no sample preparation. E-Scan 655 can be used with a wide range of liquid based products including low conductivity sterile water for injection (WFI) and proteinaceous products with suspensions.
The E-Scan 655 features a fast test cycle and simple operation. Additional benefits include quick changeover and easy recipe setup to accommodate a wide range of products and applications. The offline E-Scan 655 method can be migrated from laboratory to 100% inline testing applications at high production speeds.
The E-Scan testing process uses a set of electrode probes to scan a non-conductive container that is sealed. The container material can be glass, plastic, or poly laminate. The container or package must contain liquid (minimum fill 30%). If a pinhole, crack, or other defect is present, there is a resistance differential and change in current flow indicating a breach in the container. The approximate defect location can be identified.
- Non-destructive, non-invasive, no sample preparation
- High level of repeatability and accuracy
- Effective across all parenteral products, including extremely low conductivity liquids (WFI)
- Lower voltage exposure produces no ozone, eliminating risk to the product and environment
- Listed in USP Chapter <1207> as recommended method for parenteral liquid package inspection
- Robust method and approximate 3x Signal-Noise-Ratio for a wide range of product classes and package formats
- Simplifies the inspection and validation process