- Home
- Companies
- Intecna srl
- Products
- Intecna - Model CELDAR - ...

Intecna - Model CELDAR -Electrochemical Technologies for WWT Treatment
Alongside conventional treatments for wastewater, electrochemical techniques with very satisfactory results have spread in recent years. INTECNA has studied , developed and patented some of these and this has made possible the design, construction and installation of plants in many industrial sectors. This presentation aims to give a CRITICAL overview of the main principles underlying the electrochemical water treatment systems
Basically the electrochemical techniques for wastewater treatments are four:
- DIRECT ELECTROCHEMICAL OXIDATION
- INDIRECT ELECTROCHEMICAL OXIDATION
- ELECTRO-COAGULATION
- ELECTRO-FLOTATION
Often, working with appropriate experimental methods and choice of operating conditions, it can happen that in the same process there are reactions that belong simultaneously to one or more mentioned techniques and it is the task of careful laboratory work, finding the best experimental working conditions
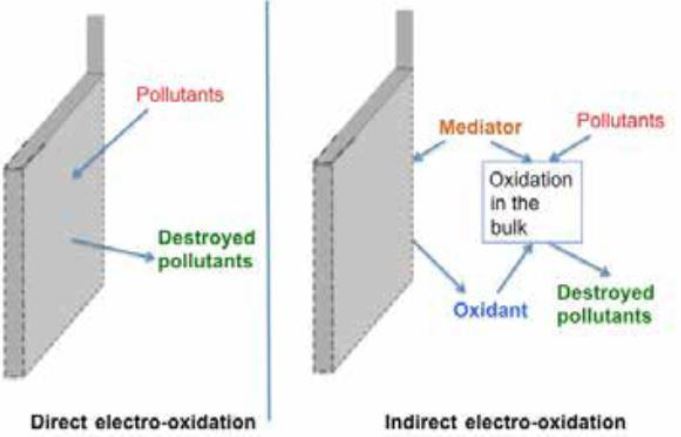
DIRECT ELECTROCHEMICAL OXIDATION
Electro-oxidation of organic pollutants occurs directly on oxide anodes (MOx) in which water (in alkaline or acidic media) is also partially oxidized to generate physically adsorbed active oxygen (adsorbed hydroxyl radicals, -OH). Furthermore, the adsorbed hydroxyl radicals may interact with the oxygen already present in the oxide anode with possible transition of oxygen from the adsorbed hydroxyl radical to the lattice of the oxide anode, forming a higher oxide, MOx+1 (chemisorbed active oxygen).The physically adsorbed active oxygen causes the complete combustion/oxidation of the organic compounds (R), and the chemisorbed active oxygen participates in the formation of selective oxidation products
COMMENTS :
In general, -OH radicals are more effective for organic pollutants oxidation than the O in the MOx+1. Selection of the anode material is critical for the process. Specifically, electrode materials must have a high over-potential for oxygen evolution to increase the current efficiency of the process towards the removal of the organic pollutants. Exemplary anode materials include BDD film on titanium substrate, Ti/Pb02, Granular graphite, Ir02, Ru02, Pt, and Pb02 This method is not wide applied for the high materials costs.
INDIRECT ELECTROCHEMICAL OXIDATION (IEO)
In the IEO process, strong oxidants are produced. The oxidants lead to the oxidation of the pollutant at the bulk of the solution. The most common processes are the electro- Fenton process (for organics) and the electro-chlorination process (for organics and inorganics), which rely on hydrogen peroxide and chlorine as the oxidants, respectively.
In the ELECTRO-FENTON PROCESS, Hydrogen peroxide is produced at the cathode of the cell. Hydrogen peroxide is one of the most powerful oxidants known, and through catalysis, H202 can be converted into hydroxyl radicals (-OH). In such a system, the cathodic and anodic reactions are :
- 02 + 2H+ + 2e- ========== H202
- Fe ===== Fe++ + 2 e-
The efficiency of the electro-Fenton system depends mostly on the efficiency of the cathode, typical cathodes include porous carbon with poly-tetrafluoroethylene (PTFE), carbon felt, graphite, carbon sponge, and activated carbon fibers, among others. The application of this way has been hindered due to the energy consumption and cost
In ELECTRO-CHLORINATION PROCESS, Hypochlorite, an oxidant-disinfectant, is frequently used in water treatment. From a safety and procurement point of view, electro-chlorination provides a way to generate hypochlorite in situ, this abolishes the constraints created by the need to store chlorine or to transport and store dilute hypochlorite solutions. Electro-chlorination was primarily developed to protect cooling circuits found on offshore platforms but this process has also been implemented in many other uses. The main electrochemical reactions involved in the process are the oxidation of CI- and the reduction of water at the anode and cathode of the cell, respectively:
- 2 CI - ====== Cl2 + 2 e -
- 2 H2 O + 2e- H2+20H-
If we want to summarize the effects of direct and indirect electrochemical oxidation we can see from the diagram that the first occurs directly on the surface of the electrode and the second occurs through the formation in the solution in electrolysis of an intermediate compound (Mediator).