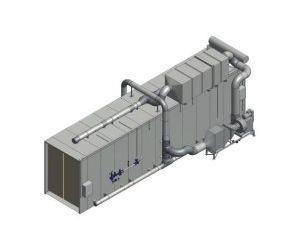
Lesni - Model ETO -Abatement Plant
Sterilisation of medical devices with a touch of excellence. The complete safe solution for all ethylene oxide process emissions. LESNI`s innovative and proven solution purifies the emissions from the sterilisation of medical devices. This delivers a safe and reliable "state of the art" system that meets the current stringent regulation with high destruction efficiency.
The recognised LESNI balancer/abator system provides a very controlled approach to handling competently hazardeous ethylene oxide gas. This secures lowest discharge emissions (currently as low as 0.5 mg/Nm³ according to TA-Luft). Lesni also design and supply quality integrated preconditioning and postconditioning cells for this industry. Energy costs are substantially reduced by the incorporation of well sized recuperative heat recovery. The Catalytic Abatement Plant "CAP" is designed to treat all potential low fugitive air extracts from degassing and aeration, as well as the vacuum pump high concentrate vents and the waste liquid seal.
Preconditioning is a necessary step in the sterilization process, which prepares products / devices ahead of sterilization and save precious time inside the sterilizer, it enhances ethylene oxide penetration into the product and packaging.
In LESNI Preconditioning Cell the products are in a temperature and humidity controlled area.
A typical range for this preconditioning step is a temperature at 40 – 60 °C and relative humidity of 50 – 65 %.
Product on pallets placed in closed, insulated chamber where air flow, temperature and relative humidity evenly distributed and maintained at pre-set values.