- Home
- Companies
- Particle Measuring Systems (PMS)
- Products
- PMS - Model APSS-2000 - Liquid Particle ...

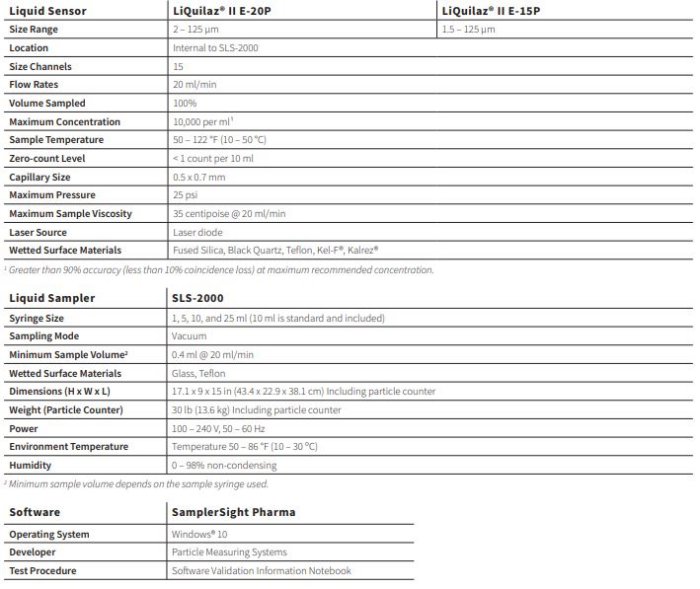
PMS - Model APSS-2000 -Liquid Particle Counter for USP 788
The APSS-2000 Liquid Particle Counter for USP 788 sizes and counts suspended particulate matter in a wide range of liquids, including parenterals, to meet all current U.S., European and Japanese Pharmacopoeia standards.
Details:
- Compliant with USP 788, USP 789, USP 729 and all equivalent European and Japanese pharmacopoeia
- Proven long term reliability
- Software database compatibility with LIMS (SQL Database)
- Multiple syringe size configurations
- Sensitivity range: 1.5 – 125 μm
- 18 configurable channels
- Cost effective
Along with USP 788 for particles in liquids, the APSS-2000 Particle Counter has recipe functions that allow it to adapt to future regulatory changes, and its small sample volume minimizes the waste of expensive product.
The APSS-2000 Liquid Particle Counter includes
- SLS-1000 Syringe Sampler
- LiQuilaz® eSeries Light Obscuration Spectrometer
- SamplerSight Pharma Software,
- reporting meets 21 CFR Part 11
SamplerSight Pharma Software allows operators to manage sampling requirements for batch-based operations and provides a comprehensive view of the batch information with histogram, time plot and tabular data presented in an easy-to-use format that is easily reported. SamplerSight Pharma features a validatable, user-friendly, Windows-based software with context-sensitive help.
Find the best particle counter from Particle Measuring Systems PMS for your application.
Particle Measuring Systems engineers, manufactures, installs, calibrates, repairs, and maintains the APSS-2000 liquid particle counter.
- Particle size capability from 1.5 to 125 µm
- Fifteen user-selectable sizing channels
- Meets and exceeds all USP, EP and JP requirements
- Sample volumes from 0.4 ml to 1 liter
- Adapters for SVI and IV bag testing
- Automated sensor calibration
- Plug and play ultrasonic bath and magnetic stirrer options available
Defect Reduction
- 100% sample volume for greater accuracy
- A precise sample volume within 0.4 ml is achieved with the syringe sampler, producing repeatable results
- SamplerSight Pharma software comes with a Validation Information Notebook, providing comprehensive software development
- documentation and full IQ, OQ, and PQ protocols
- SamplerSight Pharma complies with FDA 21 CFR 11
User-Friendly
- Reduce operator error and ensure process accuracy with recipes for repeat sample processing to USP, EP, or JP standards
- Automated particle counter calibration wizard for full calibration or routine verification
- Alarm levels for pass/fail criteria ensure quality control
- Automated backup scheduling ensures enhanced data integrity
Comprehensive System
- Easy-to-use pull-down menus
- Active Directory security password maintains system integrity
- Particle measurements are reported per ml or per container values
- Small sample volume minimizes waste of expensive product
- Various custom report formats are available
- Electronic report approval
- Pharmaceutical parenteral monitoring to U.S., EP, JP and FDA standards
- Parts/medical device cleanliness testing
- Laboratory water sampling for purified water testing and water for injection (WFI)
- Filter efficiency testing