Particle Measuring Systems (PMS)
- Home
- Companies
- Particle Measuring Systems (PMS)
- Products
- PMS - Model Lasair Pro - Cleanroom ...

PMS - Model Lasair Pro -Cleanroom Particle Counter
The top choice for non-viable cleanroom monitoring and certification. The Lasair Pro particle counter is the standard in cleanroom monitoring with its portability and high-accuracy ensuring cleanroom compliance. With real-time information at your fingertips, the Lasair Pro delivers accurate data that is easy to export and manage.
Most popular related searches
particle counter
cleanroom particle counting
cleanroom particle
contamination monitoring
contaminant monitoring
cleanroom monitoring
high-pressure diffuser
contaminant monitoring system
contamination monitoring system
cleanroom air
- Lightweight and portable
- Easily transfer data to your system via Ethernet, WiFi, serial or analog connections
- Meets all regulatory requirements including EU GMP Annex 1, ISO 14644, and 21 CFR Part 11
- All supported by Particle Measuring Systems’ expert teams including global service, advisory, and sales
Product Highlights:
- Versatile Applications: The Lasair Pro supports cleanroom certification and clean area monitoring, ensuring compliance with global data integrity standards.
- Advanced Features: Designed for air and gas particle counting, it meets EU GMP Annex 1 and 21 CFR Part 11, with local and remote control for flexible sampling.
- Straightforward Data Management and Robust Security: Three user access levels, an Audit Trail with 30,000 entry storage, and multiple secure data transfer options ensure compliance with data integrity standards.
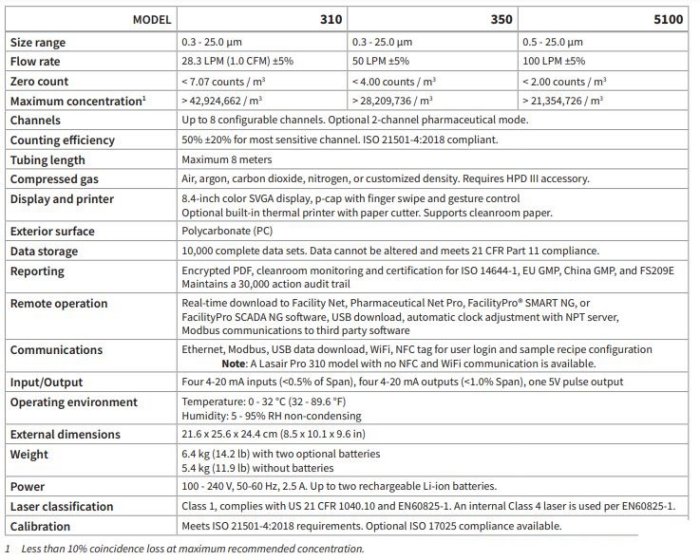
- Compressed Gas Compatibility: Monitors various gases (air, Ar, CO2, N2) with the HPD III high-pressure diffuser, and is available in three flow rates (25, 50, 100 LPM).
- User-Friendly Design: Its touchscreen interface, intuitive navigation, and sanitizable exterior make it simple and safe for cleanroom environments.
- Comprehensive PMS Support: Benefit from expert service, application guidance, and in-depth knowledge ensuring optimal performance and compliance for your contamination monitoring needs.
The Lasair Pro clean room air and gas particle counter is part of the PMS PRO Series, a complete suite of contamination monitoring tools built on decades of expertise, ensuring compliance and control in pharmaceutical manufacturing.
- 8.4-inch SVGA color touchscreen compatible with gloves
- Multi-color status lights
- 21 CFR Part 11 compliant audit trail with 30,000 recorded activities
- Up to 100 configurable users with three (3) privilege groups (administrator, supervisor, operator)
- Unlimited users with NCF use
- Up to 1,000 configurable locations organized by area, room, and location hierarchy
- Up to 100 configurable recipes
- Rechargable lithium ion batteries for portable use
- Optional thermal printer
- Portable, continuous operation
- Easy to clean polycarbonate exterior
- Reduced operator errors with pre-set recipes
- Unlimited users and recipes configurable with NFC access cards
- Long-term data archive with DataAnalyst software
- VHP-resistant enclosure and HEPA-filtered exhaust
- Audit trail meets latest data integrity requirements
- Comprehensive validation manual (optional)
- Cleanroom monitoring
- Facility certification for ISO 14644-1:2015, EU GMP, China GMP, FS 209E
- Trend analysis
- Statistical process control
- Troubleshooting particle excursions
- Manifold compatible
- Portable or dedicated use